I am frequently asked about the cause of those wear and tear changes we see in the tendons of the rotator cuff and elsewhere in the body. In general, I explain it is a result of those age related changes that we all experience in life. A study below explores that topic further and may shed some light on why some patients who may not be active develop tendon problems. It is translated from orthopedics to English below.
Tendinopathy
Conventional thinking says that tendinopathy—defined as chronic tendon degeneration—is due to tendon overload, leading to microscopic collagen fiber failure and a failed healing response. It also says that inflammation is not part of the pathologic process, because inflammatory cells are not seen in biopsies obtained at the time of surgery in patients with tendinosis.
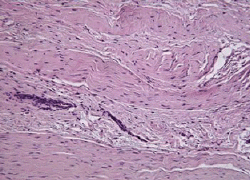 |
|
Fig. 1 Histologic appearance of tendinosis tissue shows a characteristic pattern of fibroblasts and vascular, atypical, granulation-like tissue. Courtesy of Scott A. Rodeo, MD
|
Tendon overload vs. “underload”
Stem Cells
Stem cells may also play a role in the development of tendinopathy. Tendon stem cells can differentiate into tenocytes, which lead to tendon repair, or into osteocytes or adipocytes. Researchers have found that treating tendon stem cell cultures with prostaglandin E2 (PGE2) induces both adipogenesis and osteogenesis. As a result, the number of tenocytes is reduced and fatty and calcified tissues are produced, as seen in tendinopathy. An analysis of the effect of mechanical load on tendon stem cells found that when tendon stem cells were stretched, they could continue to differentiate into tenocytes with 4 percent strain. At an 8 percent strain, however, some of the cells differentiated into adipogenic, chondrogenic, and osteogenic lineages. “So, mechanical load clearly plays a role in these pathways,” he concluded.
Inflammatory Mediators
The expression of inflammatory mediators may occur in the early stages of tissue injury. MMPs play an important role in tissue degradation and matrix remodeling and that inflammatory mediator expression can increase MMP activity. Imbalances between MMPs and their inhibitors have been implicated in the underlying origin of tendinopathy. Researchers conducted a study in which they biopsied rotator cuff synovium and bursa at the time of rotator cuff repair. They found increased expression of MMPs and inflammatory mediators. Increased synovial inflammation and tissue degradation correlated with cuff tear size.
Implications for treatment
Eccentric exercise may work via mechanical stimulation, leading to modulation of inflammatory mediators and a shift in the balance of MMPs and catabolic and anabolic gene expression. MMP inhibitors have the potential to prevent ongoing tendon degeneration. Some studies have shown that MMP inhibitors can prevent the matrix degeneration that occurs with stress deprivation in rat tail tendon. In addition, MMP inhibitors prevented loss of material properties associated with stress deprivation. Therefore, new agents that block either inflammatory mediators or MMPs may be effective in treatment of tendinosis.
Bottom line
- Recent studies have found that mechanical understimulation of tendon cells, rather than tendon overload, may cause tendinopathy and that abnormal differentiation of tendon stem cells may play a role in its development.
- Research also indicates important interactions occur among load, inflammatory mediator expression, and MMP expression at the microscopic level.
- Future studies involving the role of mechanical load may suggest ways to modulate the loading environment to stimulate tissue repair.
- MMP inhibitors may have the potential to prevent ongoing tendon degeneration.
AAOS Now
May 2011 Issue
Reference
Thanks,
JTM, MD